How Do You Know Which Lewis Structure Is Best
According to my 9th grade textbook a Lewis structure is just a Kekule structure except the bonds are. While Lewis structures are usefulespecially when youre learning about valence oxidation states and bondingthere are many exceptions to the rules in the real world.

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube
You should start drawing your Lewis structures with a single bond and then fill the octets.

. Count the Total Number of Valence Electrons. They are separated by a double-headed arrow two arrows are used for equilibrium. You do not need to provide the Lewis structure again.
Determine the total number of valence electrons in a molecule 2. This is called the octet rule Count all the valence electrons from the atoms involved in your structure. Its essential for predicting molecular geometry molecule polarity and reactivity in a compound.
Lewis Structures Vs. The first step is determining the count of valence electrons found in all the atoms that make the molecule you are dealing with. In simple molecules the atom with the most available sites for bondng is usually placed central.
Draw the Lewis Dot Structure for the Hydrogen atom. Draw a skeleton for the molecule which connects all atoms using only single bonds. We will use this later in the class to make very reactive reagents.
You have determined the best Lewis structure octets completed and lowest formal charges for NO 3- but there are a number of ways to show this structure. What is the best Lewis structure. The O-O single bond is very weak about 13 of a C-C and will break very readily.
However atoms can and do form molecules that are not ideally stable. Octet ruleAtoms lose gain or share electrons in order to have a full valence shell of eight electrons. Drawing Lewis structures with O-O single bonds because these structures are very unstable.
Generally electrons are paired. Begin drawing the Lewis dot structure of the molecule. Start with the components of the molecule atoms polyatomics and count the valence electrons available then place them where they need to go to fulfill octet charge balance and electro negativity preferences.
Indicate which N-O bond s are 118 pm and which N-O bond s are 136 pm in the bonding framework below. LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1. 118 pm and 136 pm.
An outline of how to detemine the best Lewis structure for an example NO 3-is given below. The best lewis structure is B Lets have a look at the rules how we set up lewis structures most atoms want to have 8 electrons or 2 in the outer shell the valence shell. Provide the best Lewis structure by drawing in any missing multiple bonds and lone pairs.
Remember resonance structures have the same placement of atoms meaning that they represent the same compound and only the arrangement of electrons is different. Drawing Lewis structures where one or more atoms does not have a full valence shell. All valence electrons of the atoms in Lewis structures must be shown.
It will also work with more complex molecules and ions if you recognize that individual atoms will have the same arrangement of bonds and lone pairs as they do in the simple structures. Formal chargeThe charge assigned to an atom in a molecule assuming that electrons in a chemical bond are shared equally between atoms. If they are full and you still have electrons that need to be added then add a double bond and try to use the electrons that you need to.
If a molecule has more than one Lewis structure it can be represented by the corresponding resonance forms. This helps determine which of a few Lewis structures is most correct. So the best Lewis structure has a NO double bond with two lone pairs on oxygen and a lone pair and one unpaired electron on nitrogen.
Draw the Lewis Structure. How to draw Lewis Diagrams. The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs.
See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding. The following is a general procedure for drawing Lewis structures. How to Draw Lewis Structures.
With these two questions we can determine whether this molecule is a lewis base or acid or you can always draw a Lewis Dot structure So Al is the central atom which has 3 valence electrons and there are no unbonded electrons as one of each is bonded to each Br however the valence shell of the central atom is not full therefore this molecule can act as a. For those who are concerned about how to draw Lewis structures below is a simple procedure on how to do it. The response to this question depends on how you interpret the term best As described on page 73 of the course reading the lewis structure with formal charges closest to 0 is best in the sense that it makes a greater contribution to the resonance mixture than the other two resonance structures.
Need help with chemistry. Atoms seek to fill or half-fill their valence electron shell. Unpaired electrons are observed in odd electron molecules such as NO and NO2.
If you cant fill the octets due to a lack of electrons then try using a double bond and go from there. The video covers the basic Lewis structures youll see in an introductory chemistry class. This type of Lewis dot structure is represented by an atomic symbol and a series of dots.
You may need a periodic table for this. A video tutorial for how to draw Lewis Structures in five steps. Although it is most common to use a line to indicate a bonding pair of electrons they can be shown as electrons see the left most image below.
Divide this number by 2 to obtain the number of electron pairs. Lewis dot structures can be drawn to show the valence electrons that surround an atom itself. The N-O bond lengths in dinitrogen pentoxide have two values.

Lewis Structure Of Po3 1 Simple Method For Lewis Electron Dot Structures Context Clues Worksheets Lewis High School Chemistry
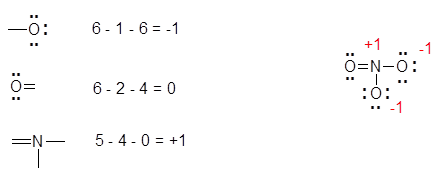
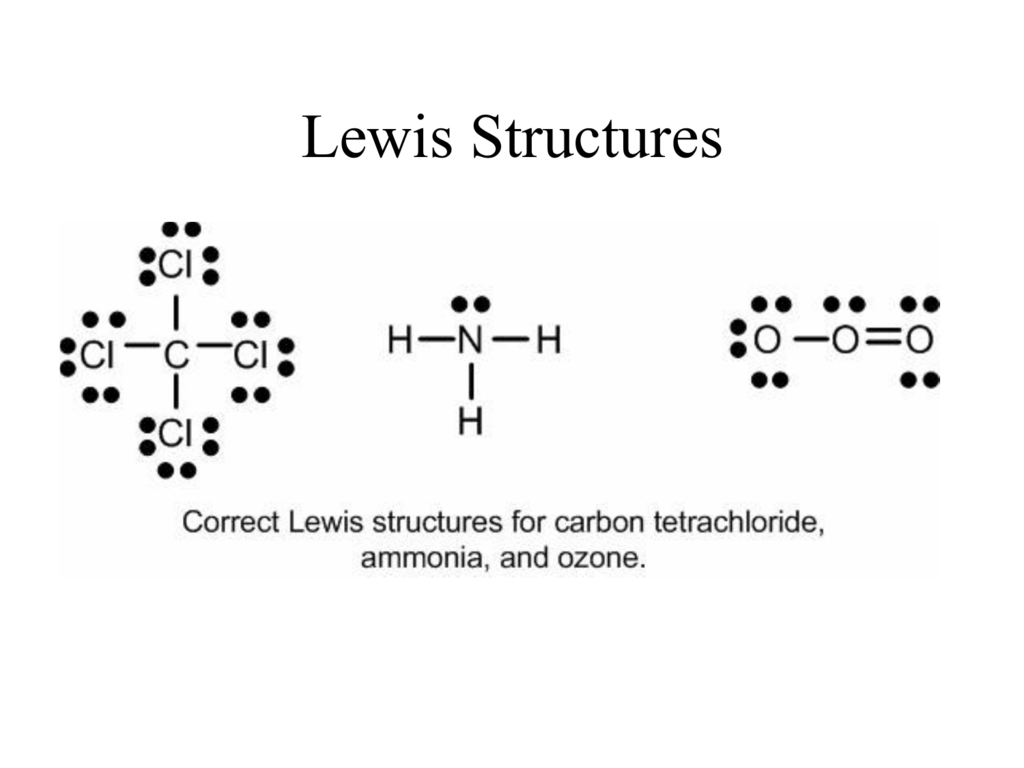
Lewis Structures Chemistry Libretexts

Drawing Lewis Structures Resonance Structures Chemistry Tutorial Youtube Chemistry Science Education Organic Chemistry

Choose The Best Lewis Structure For Ch2cl2 In 2022 Lewis Best Chosen

Best Chemistry App To Learn Chemistry From Basic To Advance Youtube How To Learn Chemistry Chemistry Basics Chemistry Education

Pocl3 Lewis Structure Phosphoryl Chloride Math Lewis Molecules

How To Draw Lewis Structures Youtube

Ocl2 Lewis Structure Dichlorine Monoxide Molecules Lewis Electrons

Xef2o Lewis Structure Xenon Oxytetrafluoride Molecules Lewis Biology

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube Molecular Geometry Molecular Chemistry

Pcl3 Lewis Structure Phosphorus Trichloride Math Lewis Molecules

Help Students Understand How To Draw Lewis Structures Understand Molecular Geometry And The Polar Nature Of Ionic And Molecular Shapes Vsepr Theory Molecular



Comments
Post a Comment